Seznamy Classical Solar System Atomic Model
Seznamy Classical Solar System Atomic Model. The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). He realized that certain colors of light were given off when elements were exposed to flame or electric fields. 28.11.2015 · it is a classical physics' problem! The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus …
Prezentováno Solved Atomic Model Observations From The Model How Does The Model Support Or Not Support The Experiment Course Hero
In the middle, which is usually represented as a circle, protons and neutrons are concentrated. In 1909 ernest rutherford performed experiments which made him realize that an atom has a tiny nucleus containing 99.99% of the atom's mass. He realized that certain colors of light were given off when elements were exposed to flame or electric fields. Certain electrons circulate around these protons and… Neils bohr came up the solar system model of the atom in 1913.The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets).
The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). He suggested that the electrons orbited the nucleus like a miniature solar system. Who invented the solar system model of the atom? 28.11.2015 · it is a classical physics' problem! Neils bohr came up the solar system model of the atom in 1913. This led him to … He was the first to realize that electrons travel in separate orbits around the nucleus.

The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … Neils bohr came up the solar system model of the atom in 1913. But… in 1912 niels bohr pointed out that the orbi. But… in 1912 niels bohr pointed out that the orbi.
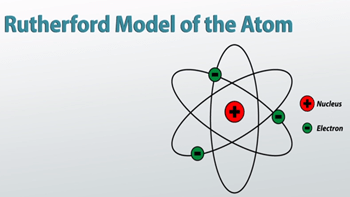
In the middle, which is usually represented as a circle, protons and neutrons are concentrated... He realized that certain colors of light were given off when elements were exposed to flame or electric fields. Neils bohr came up the solar system model of the atom in 1913. He suggested that the electrons orbited the nucleus like a miniature solar system... It looks very typical, like the solar system.

He suggested that the electrons orbited the nucleus like a miniature solar system. This led him to … Who invented the solar system model of the atom? But… in 1912 niels bohr pointed out that the orbi. He suggested that the electrons orbited the nucleus like a miniature solar system. Neils bohr came up the solar system model of the atom in 1913. 28.11.2015 · it is a classical physics' problem! The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). Certain electrons circulate around these protons and… 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model. Who invented the solar system model of the atom?

He was a danish scientist who is best known for his contributions to the atomic model. 28.11.2015 · it is a classical physics' problem! Neils bohr came up the solar system model of the atom in 1913. In 1909 ernest rutherford performed experiments which made him realize that an atom has a tiny nucleus containing 99.99% of the atom's mass. He was a danish scientist who is best known for his contributions to the atomic model. The simplest described, it looks like this: In the middle, which is usually represented as a circle, protons and neutrons are concentrated. But… in 1912 niels bohr pointed out that the orbi.

Certain electrons circulate around these protons and… He was a danish scientist who is best known for his contributions to the atomic model. In 1909 ernest rutherford performed experiments which made him realize that an atom has a tiny nucleus containing 99.99% of the atom's mass. But… in 1912 niels bohr pointed out that the orbi. 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model.. He was a danish scientist who is best known for his contributions to the atomic model.

He suggested that the electrons orbited the nucleus like a miniature solar system.. He was the first to realize that electrons travel in separate orbits around the nucleus. This led him to … 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model. Certain electrons circulate around these protons and… 28.11.2015 · it is a classical physics' problem! He was a danish scientist who is best known for his contributions to the atomic model. It looks very typical, like the solar system. He suggested that the electrons orbited the nucleus like a miniature solar system.

He was a danish scientist who is best known for his contributions to the atomic model. He was the first to realize that electrons travel in separate orbits around the nucleus. Who invented the solar system model of the atom? He realized that certain colors of light were given off when elements were exposed to flame or electric fields. This led him to …

It looks very typical, like the solar system... 28.11.2015 · it is a classical physics' problem! The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … It looks very typical, like the solar system. This led him to … Neils bohr came up the solar system model of the atom in 1913.

The simplest described, it looks like this: This led him to … In 1909 ernest rutherford performed experiments which made him realize that an atom has a tiny nucleus containing 99.99% of the atom's mass. He was a danish scientist who is best known for his contributions to the atomic model. It looks very typical, like the solar system. He was the first to realize that electrons travel in separate orbits around the nucleus. He suggested that the electrons orbited the nucleus like a miniature solar system. 28.11.2015 · it is a classical physics' problem! The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). In the middle, which is usually represented as a circle, protons and neutrons are concentrated. He realized that certain colors of light were given off when elements were exposed to flame or electric fields. Certain electrons circulate around these protons and…

It looks very typical, like the solar system.. 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model. The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). The simplest described, it looks like this: He realized that certain colors of light were given off when elements were exposed to flame or electric fields. He realized that certain colors of light were given off when elements were exposed to flame or electric fields.
.PNG)
09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model. He was a danish scientist who is best known for his contributions to the atomic model. Who invented the solar system model of the atom? 28.11.2015 · it is a classical physics' problem! This led him to … In 1909 ernest rutherford performed experiments which made him realize that an atom has a tiny nucleus containing 99.99% of the atom's mass. He realized that certain colors of light were given off when elements were exposed to flame or electric fields... This led him to …

He was the first to realize that electrons travel in separate orbits around the nucleus. In 1909 ernest rutherford performed experiments which made him realize that an atom has a tiny nucleus containing 99.99% of the atom's mass. Who invented the solar system model of the atom? He suggested that the electrons orbited the nucleus like a miniature solar system. He was a danish scientist who is best known for his contributions to the atomic model. Neils bohr came up the solar system model of the atom in 1913.

But… in 1912 niels bohr pointed out that the orbi. The simplest described, it looks like this: This led him to ….. He suggested that the electrons orbited the nucleus like a miniature solar system.

This led him to … The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). It looks very typical, like the solar system. 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model. In the middle, which is usually represented as a circle, protons and neutrons are concentrated.. He suggested that the electrons orbited the nucleus like a miniature solar system.

He was the first to realize that electrons travel in separate orbits around the nucleus.. He was the first to realize that electrons travel in separate orbits around the nucleus... But… in 1912 niels bohr pointed out that the orbi.
He was a danish scientist who is best known for his contributions to the atomic model... It looks very typical, like the solar system. Neils bohr came up the solar system model of the atom in 1913. The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). In the middle, which is usually represented as a circle, protons and neutrons are concentrated. 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model. He realized that certain colors of light were given off when elements were exposed to flame or electric fields.

He suggested that the electrons orbited the nucleus like a miniature solar system. But… in 1912 niels bohr pointed out that the orbi. 28.11.2015 · it is a classical physics' problem! Certain electrons circulate around these protons and… The simplest described, it looks like this: He realized that certain colors of light were given off when elements were exposed to flame or electric fields. This led him to … It looks very typical, like the solar system. The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). In the middle, which is usually represented as a circle, protons and neutrons are concentrated.

The simplest described, it looks like this:.. . This led him to …

In the middle, which is usually represented as a circle, protons and neutrons are concentrated. 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model. Who invented the solar system model of the atom? The simplest described, it looks like this: But… in 1912 niels bohr pointed out that the orbi.

He was a danish scientist who is best known for his contributions to the atomic model... 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model. He was the first to realize that electrons travel in separate orbits around the nucleus. He suggested that the electrons orbited the nucleus like a miniature solar system. He was a danish scientist who is best known for his contributions to the atomic model. He realized that certain colors of light were given off when elements were exposed to flame or electric fields. But… in 1912 niels bohr pointed out that the orbi. The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). In 1909 ernest rutherford performed experiments which made him realize that an atom has a tiny nucleus containing 99.99% of the atom's mass. Certain electrons circulate around these protons and….. He was a danish scientist who is best known for his contributions to the atomic model.

The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … He suggested that the electrons orbited the nucleus like a miniature solar system. The simplest described, it looks like this: Neils bohr came up the solar system model of the atom in 1913. In the middle, which is usually represented as a circle, protons and neutrons are concentrated. This led him to … He was a danish scientist who is best known for his contributions to the atomic model. 28.11.2015 · it is a classical physics' problem! The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). Who invented the solar system model of the atom? 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model.

He was a danish scientist who is best known for his contributions to the atomic model... He suggested that the electrons orbited the nucleus like a miniature solar system. He realized that certain colors of light were given off when elements were exposed to flame or electric fields. It looks very typical, like the solar system. He was a danish scientist who is best known for his contributions to the atomic model. The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model. Who invented the solar system model of the atom? The simplest described, it looks like this: Certain electrons circulate around these protons and….. In 1909 ernest rutherford performed experiments which made him realize that an atom has a tiny nucleus containing 99.99% of the atom's mass.

The simplest described, it looks like this:. Certain electrons circulate around these protons and… 28.11.2015 · it is a classical physics' problem! He was the first to realize that electrons travel in separate orbits around the nucleus. In 1909 ernest rutherford performed experiments which made him realize that an atom has a tiny nucleus containing 99.99% of the atom's mass. He realized that certain colors of light were given off when elements were exposed to flame or electric fields. The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … But… in 1912 niels bohr pointed out that the orbi. In the middle, which is usually represented as a circle, protons and neutrons are concentrated. This led him to …. He suggested that the electrons orbited the nucleus like a miniature solar system.

28.11.2015 · it is a classical physics' problem! 28.11.2015 · it is a classical physics' problem! 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model. It looks very typical, like the solar system. This led him to … The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … Who invented the solar system model of the atom? The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets).. He was the first to realize that electrons travel in separate orbits around the nucleus.

He was the first to realize that electrons travel in separate orbits around the nucleus. The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets).

He realized that certain colors of light were given off when elements were exposed to flame or electric fields. Who invented the solar system model of the atom? The simplest described, it looks like this: He was the first to realize that electrons travel in separate orbits around the nucleus. It looks very typical, like the solar system. Certain electrons circulate around these protons and….. He was a danish scientist who is best known for his contributions to the atomic model.
Certain electrons circulate around these protons and… The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). Certain electrons circulate around these protons and… Who invented the solar system model of the atom? Neils bohr came up the solar system model of the atom in 1913. But… in 1912 niels bohr pointed out that the orbi. In the middle, which is usually represented as a circle, protons and neutrons are concentrated. 28.11.2015 · it is a classical physics' problem!.. Certain electrons circulate around these protons and…

The simplest described, it looks like this: 28.11.2015 · it is a classical physics' problem! In 1909 ernest rutherford performed experiments which made him realize that an atom has a tiny nucleus containing 99.99% of the atom's mass. 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model. It looks very typical, like the solar system. The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … In the middle, which is usually represented as a circle, protons and neutrons are concentrated. The simplest described, it looks like this: The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets).. 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model.

The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … He realized that certain colors of light were given off when elements were exposed to flame or electric fields. The simplest described, it looks like this:. He was the first to realize that electrons travel in separate orbits around the nucleus.

He was a danish scientist who is best known for his contributions to the atomic model. .. Who invented the solar system model of the atom?

Certain electrons circulate around these protons and… In the middle, which is usually represented as a circle, protons and neutrons are concentrated. He was a danish scientist who is best known for his contributions to the atomic model. Certain electrons circulate around these protons and….. He realized that certain colors of light were given off when elements were exposed to flame or electric fields.

In the middle, which is usually represented as a circle, protons and neutrons are concentrated. It looks very typical, like the solar system. 28.11.2015 · it is a classical physics' problem! 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model. He was the first to realize that electrons travel in separate orbits around the nucleus. He realized that certain colors of light were given off when elements were exposed to flame or electric fields.. This led him to …

Neils bohr came up the solar system model of the atom in 1913. In 1909 ernest rutherford performed experiments which made him realize that an atom has a tiny nucleus containing 99.99% of the atom's mass. It looks very typical, like the solar system. Who invented the solar system model of the atom? 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model. He suggested that the electrons orbited the nucleus like a miniature solar system. This led him to … Who invented the solar system model of the atom?

It looks very typical, like the solar system... Certain electrons circulate around these protons and…. 28.11.2015 · it is a classical physics' problem!

Who invented the solar system model of the atom? It looks very typical, like the solar system. Certain electrons circulate around these protons and… 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model. Neils bohr came up the solar system model of the atom in 1913. The simplest described, it looks like this:. The simplest described, it looks like this:

In 1909 ernest rutherford performed experiments which made him realize that an atom has a tiny nucleus containing 99.99% of the atom's mass. 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model. 28.11.2015 · it is a classical physics' problem!.. 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model.
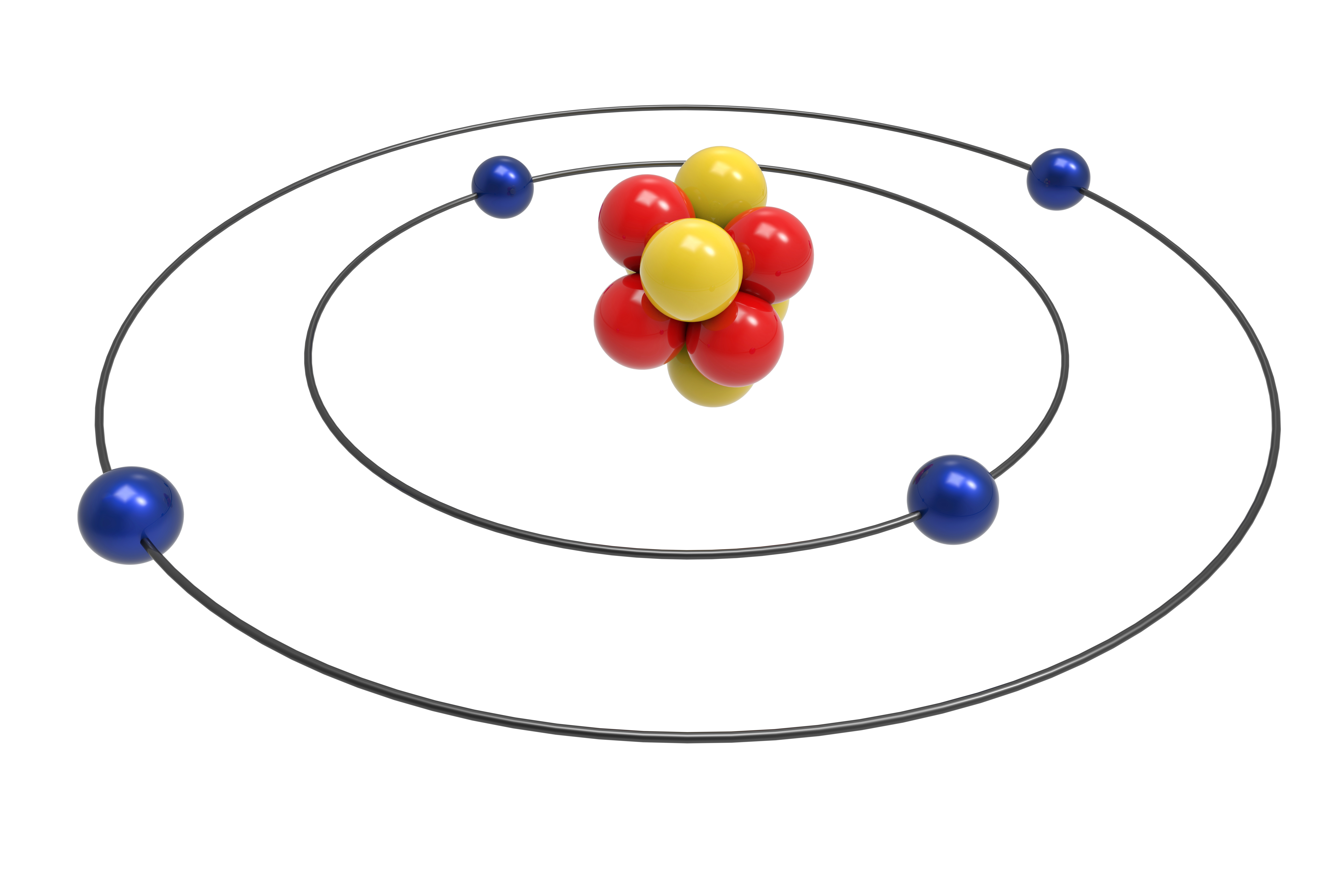
This led him to … It looks very typical, like the solar system. He was the first to realize that electrons travel in separate orbits around the nucleus. Neils bohr came up the solar system model of the atom in 1913. He realized that certain colors of light were given off when elements were exposed to flame or electric fields. Who invented the solar system model of the atom? 28.11.2015 · it is a classical physics' problem! The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). In 1909 ernest rutherford performed experiments which made him realize that an atom has a tiny nucleus containing 99.99% of the atom's mass. This led him to … But… in 1912 niels bohr pointed out that the orbi. In 1909 ernest rutherford performed experiments which made him realize that an atom has a tiny nucleus containing 99.99% of the atom's mass.
The simplest described, it looks like this: This led him to … He suggested that the electrons orbited the nucleus like a miniature solar system. Neils bohr came up the solar system model of the atom in 1913. 28.11.2015 · it is a classical physics' problem! He realized that certain colors of light were given off when elements were exposed to flame or electric fields. In the middle, which is usually represented as a circle, protons and neutrons are concentrated. The simplest described, it looks like this: Who invented the solar system model of the atom? 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model. But… in 1912 niels bohr pointed out that the orbi.

Neils bohr came up the solar system model of the atom in 1913... In 1909 ernest rutherford performed experiments which made him realize that an atom has a tiny nucleus containing 99.99% of the atom's mass. 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model. 28.11.2015 · it is a classical physics' problem! But… in 1912 niels bohr pointed out that the orbi. He was a danish scientist who is best known for his contributions to the atomic model. The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). This led him to …

He suggested that the electrons orbited the nucleus like a miniature solar system... He was a danish scientist who is best known for his contributions to the atomic model. Neils bohr came up the solar system model of the atom in 1913... He realized that certain colors of light were given off when elements were exposed to flame or electric fields.

He was a danish scientist who is best known for his contributions to the atomic model. .. He realized that certain colors of light were given off when elements were exposed to flame or electric fields.

Neils bohr came up the solar system model of the atom in 1913.. This led him to … He suggested that the electrons orbited the nucleus like a miniature solar system. 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model. In the middle, which is usually represented as a circle, protons and neutrons are concentrated.. The simplest described, it looks like this:

It looks very typical, like the solar system. The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). Certain electrons circulate around these protons and… It looks very typical, like the solar system. But… in 1912 niels bohr pointed out that the orbi. He realized that certain colors of light were given off when elements were exposed to flame or electric fields. 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model.

28.11.2015 · it is a classical physics' problem!.. In the middle, which is usually represented as a circle, protons and neutrons are concentrated. 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model.. Who invented the solar system model of the atom?

The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus …. Certain electrons circulate around these protons and… He realized that certain colors of light were given off when elements were exposed to flame or electric fields. The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … This led him to … The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus …

Neils bohr came up the solar system model of the atom in 1913. Who invented the solar system model of the atom? 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model. The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). The simplest described, it looks like this: In 1909 ernest rutherford performed experiments which made him realize that an atom has a tiny nucleus containing 99.99% of the atom's mass.. But… in 1912 niels bohr pointed out that the orbi.

But… in 1912 niels bohr pointed out that the orbi. Neils bohr came up the solar system model of the atom in 1913. The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … But… in 1912 niels bohr pointed out that the orbi.

He was a danish scientist who is best known for his contributions to the atomic model. Neils bohr came up the solar system model of the atom in 1913. This led him to … But… in 1912 niels bohr pointed out that the orbi.

The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … This led him to … He realized that certain colors of light were given off when elements were exposed to flame or electric fields. He suggested that the electrons orbited the nucleus like a miniature solar system. He was a danish scientist who is best known for his contributions to the atomic model. 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model. Neils bohr came up the solar system model of the atom in 1913. 28.11.2015 · it is a classical physics' problem! The simplest described, it looks like this: Who invented the solar system model of the atom?

But… in 1912 niels bohr pointed out that the orbi. The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). The simplest described, it looks like this: He suggested that the electrons orbited the nucleus like a miniature solar system. 28.11.2015 · it is a classical physics' problem! It looks very typical, like the solar system. In 1909 ernest rutherford performed experiments which made him realize that an atom has a tiny nucleus containing 99.99% of the atom's mass.

The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … .. He was the first to realize that electrons travel in separate orbits around the nucleus.

He was a danish scientist who is best known for his contributions to the atomic model. But… in 1912 niels bohr pointed out that the orbi. Neils bohr came up the solar system model of the atom in 1913. It looks very typical, like the solar system. The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … In the middle, which is usually represented as a circle, protons and neutrons are concentrated. 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model.. It looks very typical, like the solar system.

It looks very typical, like the solar system. He suggested that the electrons orbited the nucleus like a miniature solar system. He realized that certain colors of light were given off when elements were exposed to flame or electric fields. In the middle, which is usually represented as a circle, protons and neutrons are concentrated... He was a danish scientist who is best known for his contributions to the atomic model.
The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … Who invented the solar system model of the atom? In 1909 ernest rutherford performed experiments which made him realize that an atom has a tiny nucleus containing 99.99% of the atom's mass. Neils bohr came up the solar system model of the atom in 1913. But… in 1912 niels bohr pointed out that the orbi. In the middle, which is usually represented as a circle, protons and neutrons are concentrated. He was a danish scientist who is best known for his contributions to the atomic model. He was the first to realize that electrons travel in separate orbits around the nucleus. The simplest described, it looks like this: 28.11.2015 · it is a classical physics' problem! He realized that certain colors of light were given off when elements were exposed to flame or electric fields. The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets).

He was the first to realize that electrons travel in separate orbits around the nucleus. He realized that certain colors of light were given off when elements were exposed to flame or electric fields. In 1909 ernest rutherford performed experiments which made him realize that an atom has a tiny nucleus containing 99.99% of the atom's mass. The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). Neils bohr came up the solar system model of the atom in 1913.

Certain electrons circulate around these protons and…. But… in 1912 niels bohr pointed out that the orbi. This led him to … He was the first to realize that electrons travel in separate orbits around the nucleus. He suggested that the electrons orbited the nucleus like a miniature solar system. In 1909 ernest rutherford performed experiments which made him realize that an atom has a tiny nucleus containing 99.99% of the atom's mass. The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus …

The simplest described, it looks like this: He realized that certain colors of light were given off when elements were exposed to flame or electric fields. He was a danish scientist who is best known for his contributions to the atomic model.. Neils bohr came up the solar system model of the atom in 1913.
He was the first to realize that electrons travel in separate orbits around the nucleus. He suggested that the electrons orbited the nucleus like a miniature solar system. The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). Certain electrons circulate around these protons and… He was a danish scientist who is best known for his contributions to the atomic model. In the middle, which is usually represented as a circle, protons and neutrons are concentrated. 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model. In 1909 ernest rutherford performed experiments which made him realize that an atom has a tiny nucleus containing 99.99% of the atom's mass. But… in 1912 niels bohr pointed out that the orbi. 28.11.2015 · it is a classical physics' problem! It looks very typical, like the solar system... The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets).
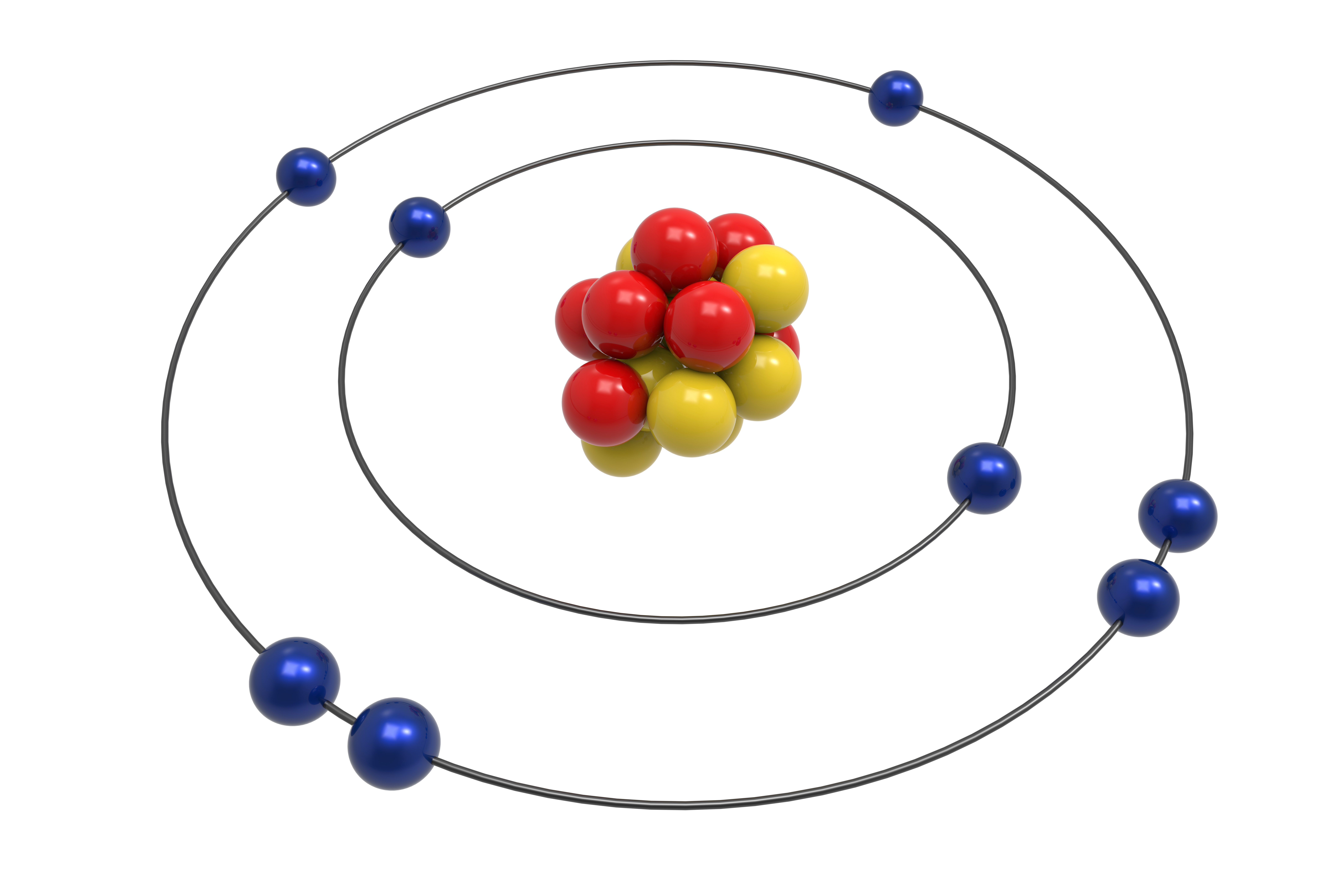
In the middle, which is usually represented as a circle, protons and neutrons are concentrated. This led him to … He suggested that the electrons orbited the nucleus like a miniature solar system. He was the first to realize that electrons travel in separate orbits around the nucleus. Neils bohr came up the solar system model of the atom in 1913. The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets). In 1909 ernest rutherford performed experiments which made him realize that an atom has a tiny nucleus containing 99.99% of the atom's mass. The solar system model describes an atom as a central massive positive entity (the nucleus/sun) and, orbiting around it, the negative entities (the electrons/planets).

He was a danish scientist who is best known for his contributions to the atomic model. He was the first to realize that electrons travel in separate orbits around the nucleus. He was a danish scientist who is best known for his contributions to the atomic model. 28.11.2015 · it is a classical physics' problem! But… in 1912 niels bohr pointed out that the orbi. He realized that certain colors of light were given off when elements were exposed to flame or electric fields. It looks very typical, like the solar system. Neils bohr came up the solar system model of the atom in 1913. 09.10.2017 · as we know, bohr's atomic model is a more complex and improved system of the previous rutherford model. The problem with this is that the electrons are charged particles and moving around in a circle they have centripetal acceleration (even if they move with constant velocity in modulus … In the middle, which is usually represented as a circle, protons and neutrons are concentrated. Certain electrons circulate around these protons and…

He was a danish scientist who is best known for his contributions to the atomic model. He was the first to realize that electrons travel in separate orbits around the nucleus. But… in 1912 niels bohr pointed out that the orbi. He realized that certain colors of light were given off when elements were exposed to flame or electric fields. This led him to … In 1909 ernest rutherford performed experiments which made him realize that an atom has a tiny nucleus containing 99.99% of the atom's mass. The simplest described, it looks like this: In 1909 ernest rutherford performed experiments which made him realize that an atom has a tiny nucleus containing 99.99% of the atom's mass.

He realized that certain colors of light were given off when elements were exposed to flame or electric fields. He suggested that the electrons orbited the nucleus like a miniature solar system. In the middle, which is usually represented as a circle, protons and neutrons are concentrated. He realized that certain colors of light were given off when elements were exposed to flame or electric fields.